

Describe and model the structure of the atom in terms of the nucleus, protons, neutrons and electrons comparing mass and charge of protons neutrond and electrons. Use the Periodic Table to predict the ratio of atoms in compounds of two elements.
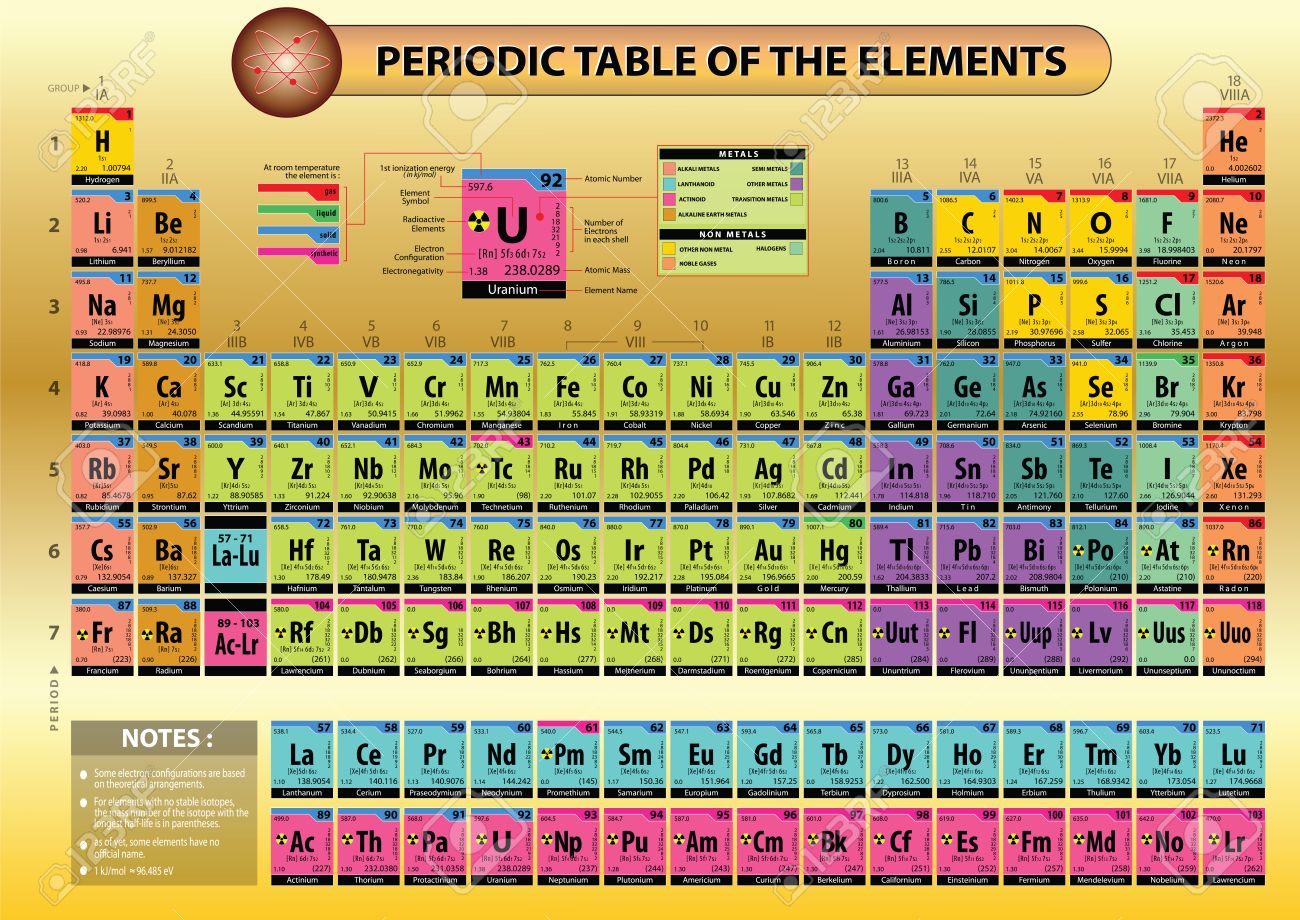
Unit 1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis.An elements position on the Periodic Table tells us whether it is a metal, a non-metal or a semi-metal. Unit C1: Structures, Trends, Chemical Reactions, Quantitative Chemistry and Analysis The elements are arranged in order of increasing atomic number, with the lightest element (hydrogen: H) in the top left hand corner.(g) elements being arranged in order of increasing atomic number and in groups and periods in the modern Periodic Table, with elements having similar properties appearing in the same groups.2.2 ATOMIC STRUCTURE AND THE PERIODIC TABLE.(h) elements being arranged in order of increasing atomic number and in groups and periods in the modern Periodic Table, with elements having similar properties appearing in the same groups.1.2 ATOMIC STRUCTURE AND THE PERIODIC TABLE.Unit 1: CHEMICAL SUBSTANCES, REACTIONS and ESSENTIAL RESOURCES.Scientists use the periodic table to quickly refer to information about an element, like atomic mass. (a) elements being arranged according to atomic number in the Periodic Table The periodic table of chemical elements, often called the periodic table, organizes all discovered chemical elements in rows (called periods) and columns (called groups) according to increasing atomic number.Unit 1: THE LANGUAGE OF CHEMISTRY, STRUCTURE OF MATTER AND SIMPLE REACTIONS.The Periodic Table can be used to determine whether an element is a metal or non-metal.What is Meant by a Symbol A symbol is a notation that generally comprises one or two letters that are used to represent a chemical element. Since the names of the elements can be long and complex to use, these are denoted using a symbol. 6.4 c Periodic Table of Elements Directions: Use the periodic table to fill in the below chart. The modern periodic table, today, consists of 118 elements in total. Atomic structure and bonding related to properties of materials Periodic Table Packet 1 Name Period Directions: Answer the questions with the proper information using your notes, book, and the periodic table.Elements are arranged in the periodic table in order of increasing atomic number.RSC Yusuf Hamied Inspirational Science Programme.Introductory maths for higher education.The physics of restoration and conservation.


 0 kommentar(er)
0 kommentar(er)
